"IProposeWe" make HOT Salt Water!
Materials:
- Some sort of Water dispensing devise (a faucet)
- At least one table spoon of salt
- A stove or hot plate, preferably a stove
Procedure:
1: Pour about 1 cup of water into your pot
2: Then turn your stove on and place the pot on top of the stove
3: In about 4 to 5 minuets take the pot off the stove.
4: You need to fill the glass 3/4 full with water.
5: Take 1 teaspoon of salt and drop it in the water
6: Stir the two together until the final solution looks clear.
7: FINALLY DRINK IT!!!!!................or not
Safety Precautions:
1: Make sure you always have a good grip on the glass, because if it drops it will shatter and break into a lot of sharp pieces.
2: Wear gloves, because when you pour the hot water into the glass you don't want the hot water to spill on your hands and burn you.
3: Make sure you do not spill the salt, because if you do you will bring bad luck on yourself, and no one wants that.....
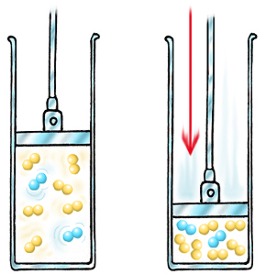
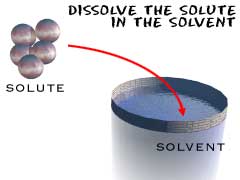
Scientific Principles: The science in this is the fact the what you end up with is a solution. The water is the
solvent and the salt is the
solute, that leaves salt water as the
solution. Stirring the salt allows the salt to diffuse throughout the water faster, and heating the water up just allows
the salt to diffuse even faster.
 I can not tell if the science in this is, because its a solution or if its because of its properties so i am going to say that its both a solution and a liquid experiment of some sort.
I can not tell if the science in this is, because its a solution or if its because of its properties so i am going to say that its both a solution and a liquid experiment of some sort.