students know that the rand
 om motion of gas molecules and their collisions with a surface create the observable pressure on that surface
om motion of gas molecules and their collisions with a surface create the observable pressure on that surfacePressure= force/area
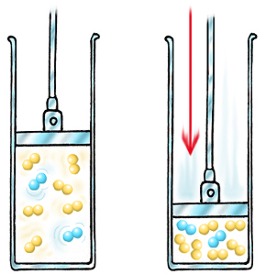
The speed and amount of gas molecules are what cause the amount of collisions on the surface. The collisions apply force to that surface. The more gas molecules in a container the more pressure, because the molecules take up more space.
The pressure of a gas is the force that the gas exerts on the walls of its
 container. When you blow air into a balloon, the balloon expands because the pressure of air molecules is greater on the inside of the balloon than the outside. Pressure is a property which determines the direction in which mass flows. If the balloon is released, the air moves from a region of high pressure to a region of low pressure.
container. When you blow air into a balloon, the balloon expands because the pressure of air molecules is greater on the inside of the balloon than the outside. Pressure is a property which determines the direction in which mass flows. If the balloon is released, the air moves from a region of high pressure to a region of low pressure.Take a soda bottle for example. When you shake the closed soda bottle the carbon dioxide is released inside the bottle. the random moving gas molecules continuously bounce and repel off each other in the small space above the liquid and the surface of the bottle. the force that is applied to each square unit is that amount of pressure that is on the bottle. You can notice that the bottle feels hard.

No comments:
Post a Comment